Join the IDMO Revolution

Cellares Announces Bristol Myers Squibb has Joined Technology Adoption Partnership Program to Evaluate Automated Manufacturing of CAR-T Cell Therapy on the Cell Shuttle Platform
- Cellares to provide proof-of-concept manufacturing for a key BMS CAR-T cell therapy
- Cellares’ proprietary Cell Shuttle is a cost-efficient, reliable, fully automated, and highly scalable manufacturing solution
- Bristol Myers Squibb is the latest company to join Cellares’ TAP program to evaluate potential benefits in meeting patient demand, lowering process failure rates, lowering manufacturing costs, and accelerating scale-out
Partnering
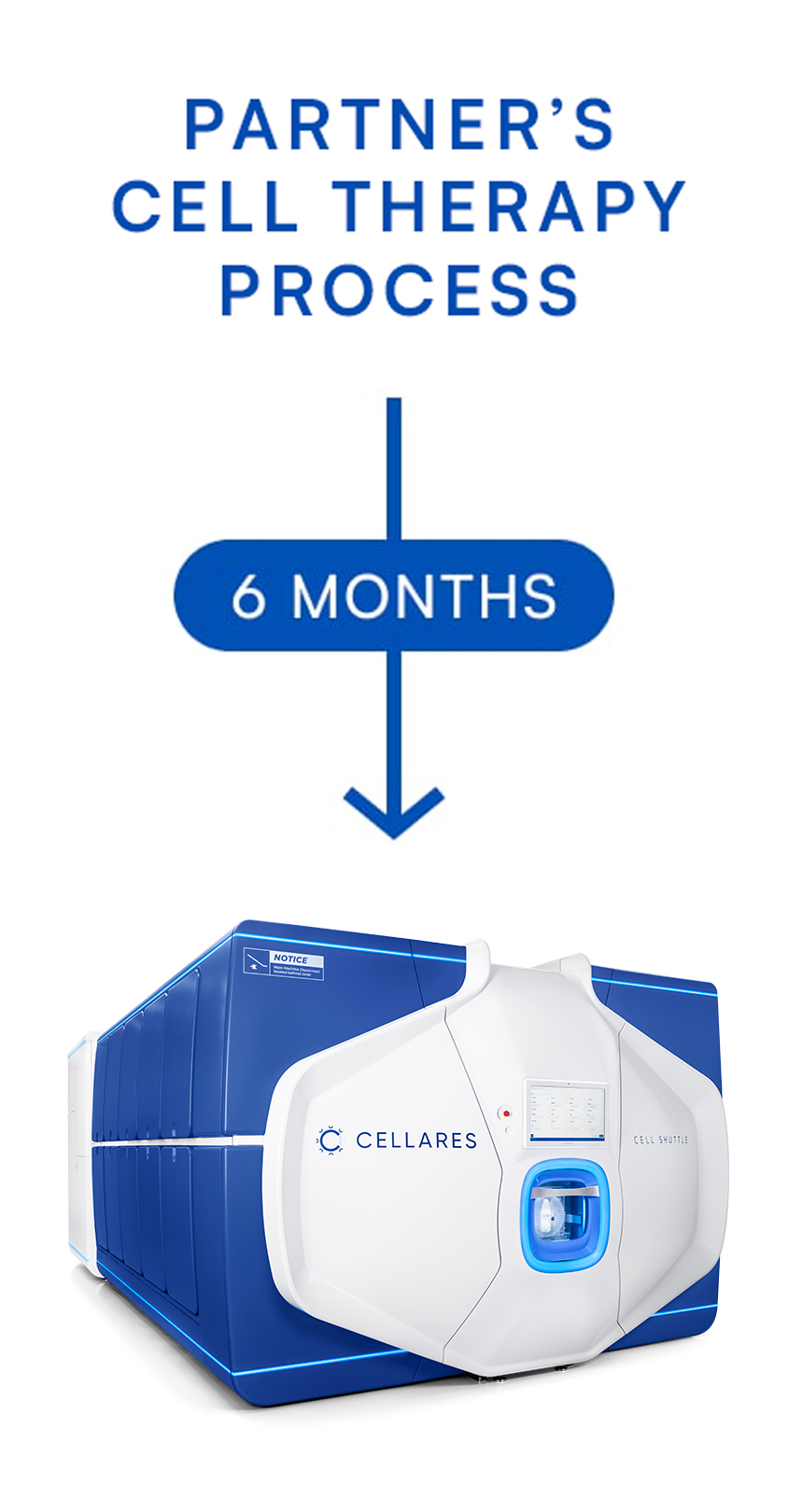
Technology Adoption Partnership (TAP)
TAP is a fast and low-risk opportunity to automate and transfer partner's cell therapy process onto the Cell Shuttle
Analyze
- Partner shares process information with Cellares
- Cellares establishes process automation plan
- Cellares provides process-specific per batch price
Transfer
- Cellares provides process-specific per batch price
- Analytical transfer as required to support automation
Automate
- Fully automated end-to-end run of the Partner’s process
- TAP data supports regulatory filings
The TAP can be completed in 6 months or less. Partnering with Cellares enables academic institutions, biotech and pharmaceutical companies to accelerate drug development and scale manufacturing, lower process failure rates, lower manufacturing costs, and meet global patient demand.
