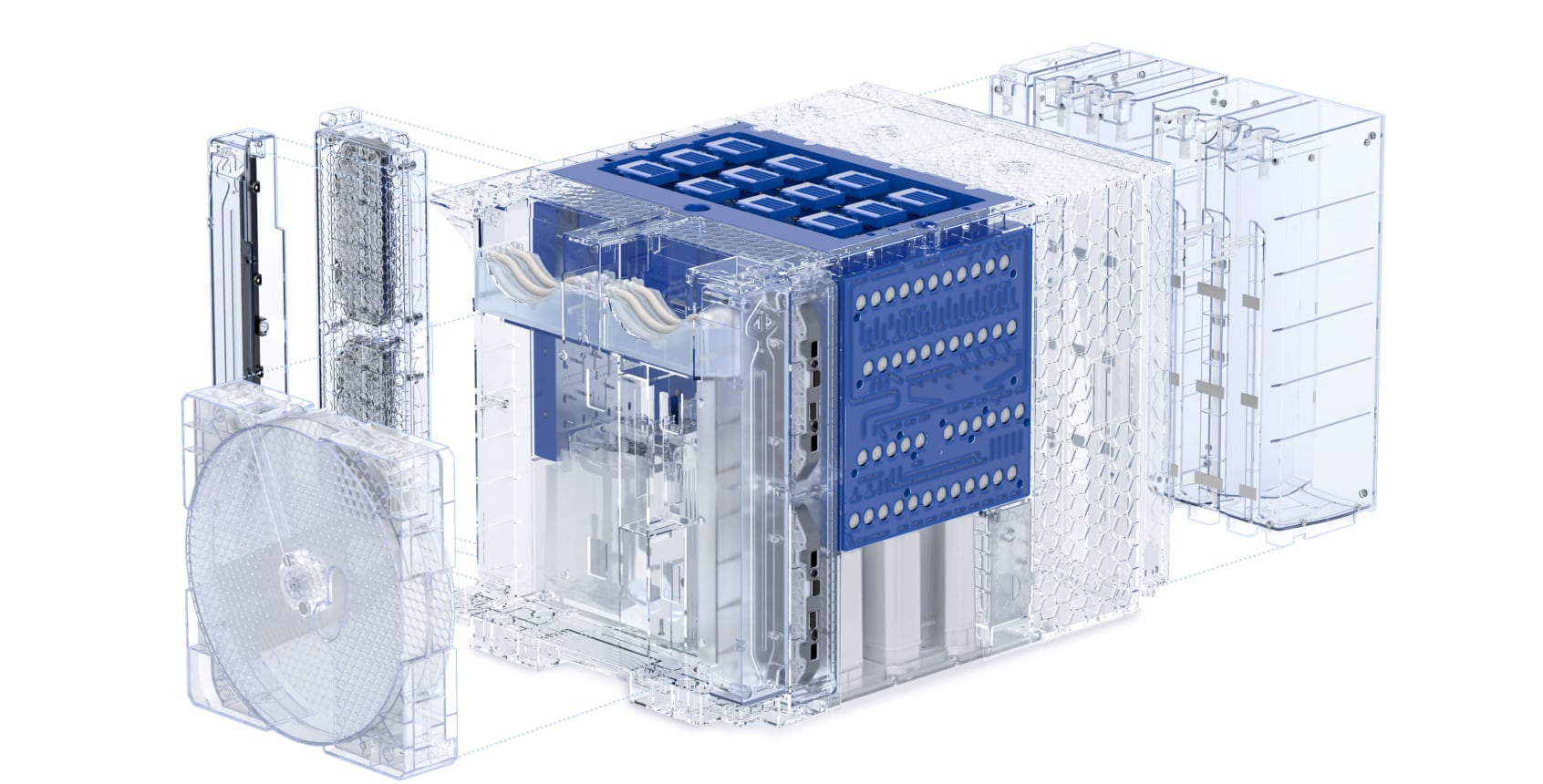
Fully Integrated, Scalable, cGMP Cell Therapy Manufacturing Platform
HIGH THROUGHPUT MANUFACTURING
Purpose-built Technology Powers the First Integrated Development & Manufacturing Organization (IDMO)

Reagent Vault System (RVS)
- Capacity for up to 200 automation-friendly reagent bottles (SLTDs)
- SLTDs come in three volumes: 10mL, 50mL, 1000mL
- Reagent agnostic (all vendors supported)
- Refrigerated at 4°C
- Just in time lane for time critical reagent delivery
- Integrated software for reagent scheduling, inventory and supply chain management
- All SLTDs undergo hydrogen peroxide decontamination cycles
Reagent Vault System (RVS)
- Capacity for up to 200 automation-friendly reagent bottles (SLTDs)
- SLTDs come in three volumes: 10mL, 50mL, 1000mL
- Reagent agnostic (all vendors supported)
- Refrigerated at 4°C
- Just in time lane for time critical reagent delivery
- Integrated software for reagent scheduling, inventory and supply chain management
- All SLTDs undergo hydrogen peroxide decontamination cycles
4x Sterile Liquid Transfer Systems (SLTS)
- Facilitates mating of Cellares’ Consumable Cartridge and automation friendly reagent bottles (SLTDs)
- Performs all sterile liquid transfer operations including reagent addition and sampling
4x Sterile Liquid Transfer Systems (SLTS)
- Facilitates mating of Cellares’ Consumable Cartridge and automation friendly reagent bottles (SLTDs)
- Performs all sterile liquid transfer operations including reagent addition and sampling
Material Handling System (MHS)
- Automates the transfer of Consumable Cartridges (CC) and automation-friendly reagent bottles (SLTDs)
- Transfers occur between feedthroughs, reagent vault system (RVS) and instruments (BPS and SLTS)
Material Handling System (MHS)
- Automates the transfer of Consumable Cartridges (CC) and automation-friendly reagent bottles (SLTDs)
- Transfers occur between feedthroughs, reagent vault system (RVS) and instruments (BPS and SLTS)
16x Bioprocessing Systems (BPS)
- Counterflow Centrifugal Elutriation (CCE)
- Magnetic Selection (MS)
- Electroporation (EP)
- Bioreactor System (BRS)
16x Bioprocessing Systems (BPS)
- Counterflow Centrifugal Elutriation (CCE)
- Magnetic Selection (MS)
- Electroporation (EP)
- Bioreactor System (BRS)
Integrated and Flexible Software Suite
- Process Design Studio supports 90% of cell therapy modalities (auto & allo) in a single cartridge design
- Integrated MES allows for batch scheduling across Cell Shuttle fleet
- Real-time process monitoring solution
- Auto-generation of electronic batch records
- Integrates with 3rd party software systems (ERP, MES, LIMS)
Integrated and Flexible Software Suite
- Process Design Studio supports 90% of cell therapy modalities (auto & allo) in a single cartridge design
- Integrated MES allows for batch scheduling across Cell Shuttle fleet
- Real-time process monitoring solution
- Auto-generation of electronic batch records
- Integrates with 3rd party software systems (ERP, MES, LIMS)
Cell Shuttle Feedthrough
- Up to 16 cartridges can be loaded asynchronously for multi-product, concurrent manufacturing
- Each cartridge can run a different cell therapy process as defined in the Process Design Studio
- Each cartridge can produce a different batch/product
- The feedthrough is the entry and exit point for all Consumable Cartridges
- All cartridges undergo hydrogen peroxide decontamination cycles
Cell Shuttle Feedthrough
- Up to 16 cartridges can be loaded asynchronously for multi-product, concurrent manufacturing
- Each cartridge can run a different cell therapy process as defined in the Process Design Studio
- Each cartridge can produce a different batch/product
- The feedthrough is the entry and exit point for all Consumable Cartridges
- All cartridges undergo hydrogen peroxide decontamination cycles
Cell Shuttle Sterility Paradigm
- Each batch/product has a dedicated set of pre-sterilized automation-friendly reagent bottles (SLTDs) and one Consumable Cartridge. Each of these consumable sets constitutes a closed system
- The Cell Shuttle maintains an internal ISO8 cleanroom environment
- The Cell Shuttle can be deployed in controlled not-classified environments (CNC)
- All consumables undergo hydrogen peroxide decontamination cycles as they enter the feedthrough or the reagent vault system (RVS)
Cell Shuttle Sterility Paradigm
- Each batch/product has a dedicated set of pre-sterilized automation-friendly reagent bottles (SLTDs) and one Consumable Cartridge. Each of these consumable sets constitutes a closed system
- The Cell Shuttle maintains an internal ISO8 cleanroom environment
- The Cell Shuttle can be deployed in controlled not-classified environments (CNC)
- All consumables undergo hydrogen peroxide decontamination cycles as they enter the feedthrough or the reagent vault system (RVS)
-
16 batches processed in parallel
-
75% fewer process failures
-
90% less labor required
-
90% less facility space required
-
The majority of modalities supported (auto & allo)

“Cellares’ revolutionary technology will be a game changer for the cell therapy industry.“
INTEGRATED AUTOMATION
Truly Closed and Automated from End-To-End
Electroporators (EP)
- Achieves world-class EP efficiency
- All EP parameters are customizable via Process Design Suite which enables EP optimization
- Supports all common genetic modification technologies (CRISPR, TALEN, ZFN) and reagents
- Ability to electroporate infinite number of cells via automated batch processing
Electroporators (EP)
- Achieves world-class EP efficiency
- All EP parameters are customizable via Process Design Suite which enables EP optimization
- Supports all common genetic modification technologies (CRISPR, TALEN, ZFN) and reagents
- Ability to electroporate infinite number of cells via automated batch processing
Peristaltic Pumps
- Multiple peristaltic pumps enable multiple process steps to run in parallel on the same cartridge
- Pump interfaces are used to facilitate sterile, metered liquid transfer between internal cartridge modules and automation friendly reagent bottles (SLTDs)
Peristaltic Pumps
- Multiple peristaltic pumps enable multiple process steps to run in parallel on the same cartridge
- Pump interfaces are used to facilitate sterile, metered liquid transfer between internal cartridge modules and automation friendly reagent bottles (SLTDs)
Fluidic Bus
- Integrated valves, sensors, fluid channels, and pumps automate all internal fluid transfers
- The Fluidic Bus enables reagent transfers from any module of the cartridge to any other module
- The modularity of the Fluidic Bus enables support for the majority of cell therapy modalities (auto & allo) within the same cartridge design
Fluidic Bus
- Integrated valves, sensors, fluid channels, and pumps automate all internal fluid transfers
- The Fluidic Bus enables reagent transfers from any module of the cartridge to any other module
- The modularity of the Fluidic Bus enables support for the majority of cell therapy modalities (auto & allo) within the same cartridge design
Reagent & Final Product Containers
- Smart containers provide precise, real-time volume tracking
- Integrated smart containers for starting material, reagent storage and final product formulation
Reagent & Final Product Containers
- Smart containers provide precise, real-time volume tracking
- Integrated smart containers for starting material, reagent storage and final product formulation
Bioreactor System (BRS)
- Supports expansion of >20 billion cells
- Perfusion-enabled stirred-tank design
- Closed loop control and real-time monitoring of temperature (T), dissolved oxygen, (DO) and acidity (pH)
Bioreactor System (BRS)
- Supports expansion of >20 billion cells
- Perfusion-enabled stirred-tank design
- Closed loop control and real-time monitoring of temperature (T), dissolved oxygen, (DO) and acidity (pH)
Sterile Liquid Transfer Ports (SLTP)
- Supports reagent additions, waste removal and automated sampling
- SLTPs on the Consumable Cartridge (CC) interface with the SLTPs on the automation-friendly reagent bottles (SLTDs)
- The Sterile Liquid Transfer System (SLTS) facilitates metered, automated, sterile liquid transfer into and out of the Consumable Cartridge
Sterile Liquid Transfer Ports (SLTP)
- Supports reagent additions, waste removal and automated sampling
- SLTPs on the Consumable Cartridge (CC) interface with the SLTPs on the automation-friendly reagent bottles (SLTDs)
- The Sterile Liquid Transfer System (SLTS) facilitates metered, automated, sterile liquid transfer into and out of the Consumable Cartridge
Magnetic Cell Sorter (MS)
- Supports magnetic selection with both microbeads and nanobeads (reagent agnostic)
- Supports both positive and negative selection
- Column-free design operates in batch mode to process any volume of cells
Magnetic Cell Sorter (MS)
- Supports magnetic selection with both microbeads and nanobeads (reagent agnostic)
- Supports both positive and negative selection
- Column-free design operates in batch mode to process any volume of cells
Counterflow Centrifugal Elutriator (CCE)
- Operates in batch mode to process any volume of cells
- Can be used at arbitrary times in the workflow as defined in the Process Design Studio
Counterflow Centrifugal Elutriator (CCE)
- Operates in batch mode to process any volume of cells
- Can be used at arbitrary times in the workflow as defined in the Process Design Studio
The integrated Consumable Cartridge reduces process failure rates by 75%
- Automation eliminates opportunities for operator error
- Closing the process eliminates opportunities for contamination
-
ENRICHMENT
-
BUFFER EXCHANGE
-
CONCENTRATION
-
SAMPLING
-
IN-PROCESS QC
-
SELECTION
-
ACTIVATION
-
TRANSDUCTION
-
TRANSFECTION
-
EXPANSION
-
FORMULATION
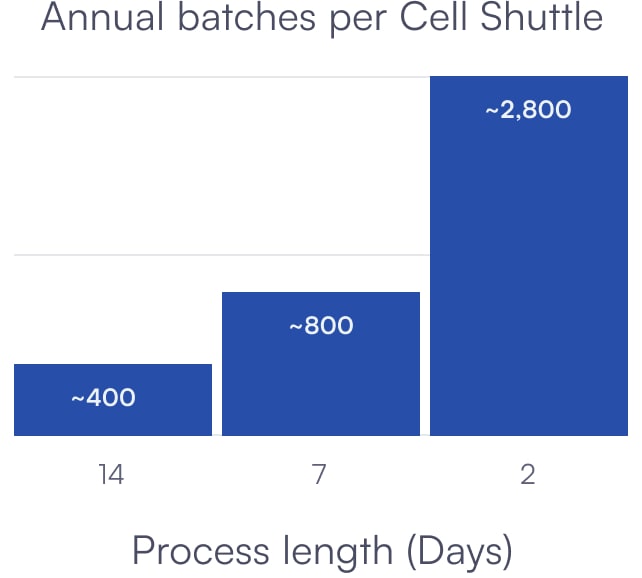
SCALABILITY
Enabling Commercial Scale Manufacturing of Cell Therapies
-

Integrated Automation
All unit operations in one Consumable Cartridge
-

High Throughput
Up to 16 cartridges for multi-product, concurrent manufacturing
-

Scalability
Up to 2,800 batches per Cell Shuttle per year

Fully Automated, Scalable, cGMP Cell Therapy QC Platform
High Throughput QC
Integrates & Automates Off-the-Shelf, Industry Standard QC Instrumentation

Modular Instrument Carts
- Integrates industry standard, off-the-shelf QC instrumentation to automate the vast majority of in-process and release assays
- Allows for interchange of instrumentation enabling customized workflows
- Minimizes workcell downtime by providing rapid replacement of pre-qualified instruments
Modular Instrument Carts
- Integrates industry standard, off-the-shelf QC instrumentation to automate the vast majority of in-process and release assays
- Allows for interchange of instrumentation enabling customized workflows
- Minimizes workcell downtime by providing rapid replacement of pre-qualified instruments
Fully Integrated IDMO Data Stack
- Supports auto-generation of electronic batch records (EBRs)
- Enables real-time manufacturing process monitoring
Fully Integrated IDMO Data Stack
- Supports auto-generation of electronic batch records (EBRs)
- Enables real-time manufacturing process monitoring
Feedthrough
- Entry point for all samples either in SLTD format or standard cryotubes
- Can process multiple samples from multiple batches concurrently
- Barcode scanning of all samples and reagents ensures maintenance of chain of identity and chain of custody
Feedthrough
- Entry point for all samples either in SLTD format or standard cryotubes
- Can process multiple samples from multiple batches concurrently
- Barcode scanning of all samples and reagents ensures maintenance of chain of identity and chain of custody
Cell Q Workcell
- Fully enclosed, cGMP QC workcell maintains sample sterility
- Hatches provide easy access for consumable and reagent addition as well as waste removal
Cell Q Workcell
- Fully enclosed, cGMP QC workcell maintains sample sterility
- Hatches provide easy access for consumable and reagent addition as well as waste removal
Integrated Liquid and Plate Handlers
- Liquid and plate handlers transfer samples and reagents between assay plates and instruments
- Automated sample handling improves reliability and reproducibility of assays via liquid volume detection and other feedback mechanisms
Integrated Liquid and Plate Handlers
- Liquid and plate handlers transfer samples and reagents between assay plates and instruments
- Automated sample handling improves reliability and reproducibility of assays via liquid volume detection and other feedback mechanisms
-
Up to 6,000 batches per year
-
Enables up to 50% lower price per batch
-
Automates the majority of cell therapy QC assays
-
Auto-generates electronic batch records
-
Pre-qualified assays accelerate analytical transfer
UNCAPPED CAPACITY
High-Throughput Manufacturing Requires High-Throughput QC
Automated High-Throughput Manufacturing

Cell Shuttles

Samples transferred via SLTDs*

Cell Q
Automated High-Throughput QC
*Automation-friendly reagent bottles
FLEXIBLE SOFTWARE
Integrated Software Suite Supports the Majority of Cell Therapy Modalities
-
SUPPORTED APPLICATIONS
- Autologous & Allogeneic
- CAR-T, TCR, HSC, NK, TIL, Treg, γδ T cells
-
FLEXIBLE SOFTWARE
- Process customization via Process Design Studio
- Enables digital process development and DOE
-
INTEGRATED HARDWARE
- Every process uses the same modular Consumable Cartridge
- No hardware customization required, which accelerates technology adoption
IDMO ADVANTAGE
Software & Hardware Integration Enable the IDMO Advantage
-
ERP
-
Cell Shuttle

-
Cell Q

-
QC LIMS
-
Electronic Batch Records
-
Scalability
Meet Total Patient Demand Globally with Unconstrained Capacity -
Cost Savings
Up to 50% Reduction in Batch Prices -
Speed
Up to 50% Faster to IND & BLA and Accelerated Global Reach